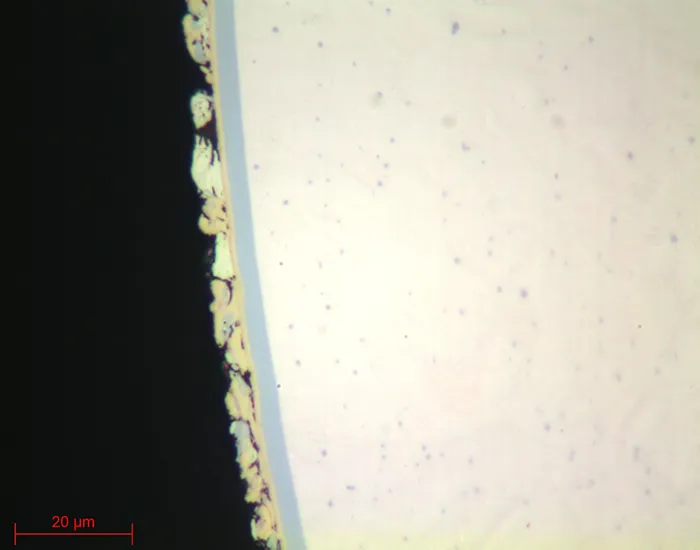
PRECIOUS METALS
QUALITY CONTROL
INTRODUCTION
The three best known precious metals are gold, silver and platinum. Palladium can be added to this list depending on the country. By extension, some metals of the platinum family are conside red precious metals such as rhodium, ruthenium, osmium and iridium.
These metals are rare due to their low presence in the earth’s crust. Precious metals have the particularity of being listed on the stock exchange. They exist therefore as investments, but also industrial raw materials.
To sum up, a metal is precious if it is rare, if its demand is strong and if its market value is high.
Symbol: Au
Atomic n°: 79
Density: 19,3
Molar mass: 197 g.mol-1
Melting point: 1063 °C
Symbol: Ag
Atomic n°: 47
Density: 10,5
Molar mass: 107,9 g.mol-1
Melting point: 961 °C
Symbol: Pt
Atomic n°: 78
Density:21,1
Molar mass: 195,1 g.mol-1
Melting point: 1770 °C
THE MAIN PRECIOUS METALS
It is chemically stable under ambient conditions. It does not oxidize in air or water. Gold is mainly used in art and jewelle ry. However, it is rarely used in its pure form in the making of jewellery.
Nowadays, gold is worked in the form of an alloy, which makes it rigid and allows its colour to be nuanced. Gold can be alloyed with silver, copper, platinum or palladium. These alloys will give different shades to the gold which will no longer be golden and bring other properties such as less ductility.
It is used in electronics for its good resistance to corrosion and its high electrical and thermal conductivity. It is notably used in microprocessors. Gold is also used in medicine, particularly in dentistry.


Silver is widely used in the field of solid metal jewellery or as a coating by electroplating. To reinforce its mechanical characteristics, silver is sometimes alloyed with copper. It can tarnish, especially in the presence of sulphide.
Silver has long been used for coinage. It is still used for this application today but mainly for collector coins or medals. Silver is often used in the field of electronics and electricity. It a very good thermal and electrical conductor.
In the field of photography, silver (in the form of silver halide) was used in the early 1990s. Now, with the development of digital cameras, it is used much less. Silver is also used in chemistry, optics, etc.


Platinum can be deposited on surfaces by electroplating. This is why in the medical field it is used to cover surgical tools or to make certain prostheses or pacemakers. Platinum can also be found in dental prostheses.
Platinum is also used in the automotive industry (for catalytic converters) together with palladium and rhodium. Platinum crucibles and pots are used in chemical and pharmaceutical laboratories.
Platinum in salt form (carboplatin and cisplatin) is used in the treatment of certain cancers. Like silver and gold, it is an essential element in the making of jewellery. In jewellery, it can be 95% pure (compared to 75% for a gold alloy). As platinum is rarer than gold, it is also much more expensive.


METALLOGRAPHIC PREPARATION
• The removal of the product to be examined (if necessary), called “CUTTING”.
• Standardisation of the geometry of the sample taken (if necessary), called “MOUNTING”.
• Improvement of the surface condition of this sample, called “POLISHING”.
• Characterisation of the sample: revealing the microstructure of the sample by an etching reagent (if necessary) called “METALLOGRAPHIC ETCHING” and microscopic observation (optical or electronic).
=> Each of these steps must be carried out rigorously, otherwise the following steps will not be pos sible.

CUTTING
In other words, it is essential to avoid heating or any deformation of the metal that could lead to strain hardening. Cutting is a fundamental step which conditions the further preparation and inspection of parts.
PRESI’s wide range of medium and large capacity cutting and micro cutting machines can be adapted to any need with regard to cutting precision, sizing or quantity of products to be cut:
Each of the cutting machines in the range is equipped with the appropriate consumables and acces sories. The clamping system and the choice of these consumables are always essential elements for the success of a metallographic cut.
=> Clamping, i.e. holding the workpiece, is also essential. Indeed, if the workpiece is not well held, the cut can present risks for the consumable, the workpiece and the machine. Figures 3 to 6 show examples of clamping for jewellery parts.
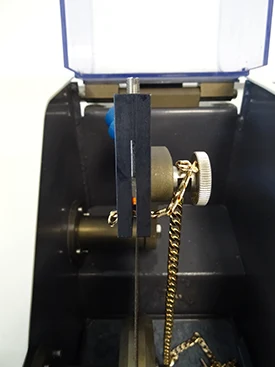


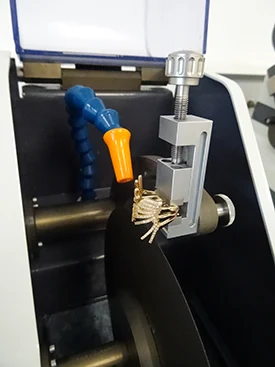
Fig. 3-4-5-6: Jewellery clamping
CONSUMABLES
 |
PRECIOUS METALS |
| Micro-cutting | S Ø180 mm UTW |
| Medium-capacity cutting | MNF |
| High-capacity cutting | MNF |
Table1: Choosing the right cut-off wheel type

MOUNTING
=> Achieving good-quality mounting is essential to protect fragile materials and also to achieve good preparation results for polishing and future analysis.
Before mounting, the specimen should be deburred with coarse abrasive paper, for example, to re- move any cutting burrs. Cleaning with ethanol (in an ultrasonic tank for even greater efficiency) is also possible. This allows the resin to adhere as well as possible to the sample and thus limits shrinkage (space between the resin and the sample).
If shrinkage persists, it can lead to problems during polishing. Abrasive grains may become lodged in this space and then be released at a later stage, thus creating a risk of pollution for the sample and the polishing surface. In this case, cleaning with an ultrasonic cleaner between each step is recommended.
There are two mounting options:
HOT MOUNTING
•Fully automatic hot-mounting press.
•Easy to use: memorisation, adjustment of processes and speed of execution make it a high-precision machine,
•The hot-mounting machine has 6 different mould diameters from 25.4-50mm.
+ POINT
COLD MOUNTING
• If the parts to be examined are fragile/sensitive to pressure
• If they have a complex geometry such as a honeycomb structure.
• If a large number of parts are to be mounted in series.
+ POINT
+ POINT
CONSUMABLES
 |
PRECIOUS METALS |
| Hot process | Phenolic Allylic glass fibre |
| Cold process | KM-U Ma2+ |
Table 2: Choosing the right mounting resin type

POLISHING
PRESI offers a wide range of manual and automatic polishing machines, with a wide choice of accessories, to cover all needs, from pre-polishing to super-finishing and polishing of single or series samples.
The MECATECH range of automatic polishers allows both manual and automatic polishing. With its advanced technologies, motor power from 750-1500 W, all the PRESI experience is concentrated in this very complete range. Whatever the sample number or size, MECATECH guarantees optimal polishing.
CONSUMABLES AND POLISHING RANGE
All the first steps of each range are called “levelling” and consist of removing material quickly to level the surface of the sample (and resin). Those given below are standard and can therefore be modified as required.
Applied pressures vary according to sample size, but in general the following applies: 1daN per 10mm mounting diameter for the pre-polishing steps (ex: Ø40mm = 4 daN) then reduce force by 0.5daN at each polishing step with an abrasive suspension.
| N° | Support | Suspension / Lubricant | Platen Speed (RPM) | Head Speed (RPM) | Rotation direction platen / head |
Time |
| 1 | P600 SiC | Ø / Water | 250 | 80 | 1’ | |
| 2 | P1200 SiC | Ø / Water | 250 | 80 | 1’ | |
| 3 | ADR II | 9μm LDP / Reflex Lub | 400 | 80 | 2’ | |
| 4 | ADR II | 3μm LDP / Reflex Lub | 400 | 80 | 2’ | |
| 5 | RFI | Alumina n°2 / Water | 200 | 80 | 1’ |
Steps 3 and 4 are made using ADRII cloth. This cloth has flexible fibres, which polish silver best.
Finally, the last step is an RFI cloth which is used with a suspension of Alumina Presi n°2. During this step, it is advisable to moisten the cloth for a few seconds beforehand. A rinse at the end of the step is preferable in order to clean the cloth and the sample from the Alumina suspension. The rotation of the head is reversed in relation to the platen in order to keep the suspension on the cloth as much as possible.
| N° | Support | Suspension / Lubricant | Platen Speed (RPM) | Head Speed (RPM) | Rotation direction platen / head | Time |
| 1 | P1200 SiC | Ø / Water | 300 | 150 | 1’ | |
| 2 | TOP | 9μm LDM / Reflex Lub | 150 | 135 | 2’ | |
| 3 | RAM | 3μm LDM / Reflex Lub | 150 | 135 | 2’ | |
| 4 | TFR | 1μm LDM / Reflex Lub | 150 | 135 | 1’ | |
| 5 | SUPRA | SPM / Water | 150 | 100 | 1’ |
| N° | Support | Support Suspension / Lubricant | Platen Speed (RPM) | Head Speed (RPM) | Rotation direction platen / head |
Time |
| 1 | P600 SiC | Ø / Water | 300 | 150 | 1’ | |
| 2 | P1200 SiC | Ø / Water | 300 | 150 | 1’ | |
| 3 | TOP | 9μm Gel 2+poly / Ø | 150 | 135 | 3’ | |
| 4 | ADR II | 3μm Gel 2+poly / Ø | 150 | 135 | 2’ | |
| 5 | NT | 1μm Gel 2+poly / Ø | 150 | 135 | 1’ | |
| 6 | SUPRA | SPM / Water | 150 | 100 | 1’ |
It continues with polishing using Gel 2+ polycrystalline diamond suspensions on polishing cloths (TOP, ADRII and NT). These suspensions contain the diamond abrasive and the lubricant, a 2-in-1 product. Their “gel” formula allows the product to remain on the polishing cloth longer.
The last step is also done with the SPM colloidal silica suspension.
| N° | Support | Suspension / Lubricant | Platen Speed (RPM) | Head Speed (RPM) | Rotation direction platen / head | Time |
| 1 | P1200 SiC | Ø / Water | 300 | 150 | 1’ | |
| 2 | TOP | 9μm LDM / Reflex Lub | 150 | 135 | 2’ | |
| 3 | RAM | 3μm LDM / Reflex Lub | 150 | 135 | 2’ | |
| 4 | NT | 1μm LDM / Reflex Lub | 150 | 135 | 1’ | |
| 5 | SUPRA | SPM / Water | 150 | 100 | 2’ |
LDM 9μm, 3μm and 1μm monocrystalline diamond suspensions are used with TOP, RAM and NT
polishing cloths.
The last step is carried out using colloidal silica suspension (SPM). This superfinishing step improves the surface finish for final observation.
DOWNLOAD THE LAB'NOTE
Simply fill out the form below:
Discover our other Lab’Note:
- 3D printing quality control
- Hardened heat treatment control
- Medical device quality control
- Steel quality control
- Stainless steel quality control
- Cast iron quality control
- Copper alloy quality control
- Aluminum quality control
- Nickel quality control
- Titanium quality control
- Ceramic materials quality control
- Electronics quality control
- Precious metal quality control